Jan uary 19
Checking the result of the whole antibiotic sensitivity experiment. Bacteria 33 has been cultured.
The process of culturing bacteria 33:
materials: two autoclaved 500ml flasks, bacteria sample, schott bottle, nutrient broth powder, sea water, inoculating loop, parafilm, ethanol, tissues, aluminium foil and permanent marker.
The original petri dish that used to grow bacteria 33 has been autoclaved/ destroyed, thus we need to get the bacteria sample from the broth. However, the chances for the bacteria to be alive in the broth is very little thus we can only hope for the best.
1. Prepare 500ml marine nutrient broth in the Schott Bottle. Autoclave it for 1 hour and 30 minutes to ensure no other living little organisms in the broth and the bottle. Once all is done, we are ready to move the broth into two autoclaved flasks.
2. Clean the laminar flow by using the usual method- spraying the ethanol on the surface and wipe it with tissues or cloth. Arrange everything nicely and put things that are needed into the clean laminar flow.
3. Open the cover of the Schott bottle and flame the mouth with fire. At the same time, open the aluminium foil of the autoclaved flask and flame the mouth with fire too. Pour 250 ml marine nutrient broth into the flask.
4. Close the mouth of the flask immediately with aluminium foil and let it cool down to room temperature. Do the same for the second flask.
5. Flame the inoculating loop with fire. Open the flask that contains
3. Because of the high contamination that has affected the results, thus decision has been made to restart doing this whole thing again by beginning from the very first step- sampling.
As Chinese New Year holidays approaching so the project will be continued after the Chinese New Year break, which is after February 5.
Tuesday, February 24, 2009
January 19- 20
Posted by SunSeT at 3:00 PM 0 comments
Thursday, February 19, 2009
January 13-16
This experiment is unlike the previous experiment, it takes about four days to complete.
Title of experiment: Antibiotic Sensitivity (Kirby-Bauer Test)
Objective: To determine the presence of antibiotic sensitivity in the MB.
Materials-
unsterilized: 7 pathogen samples, 14 petri dishes (with marine nutrient agar), parafilm, hockey stick, pipette, ampicillin
Sterilized: centrifuge tubes, tips, disk filter paper in glass petri dish, nutrient broth/agar in schott bottle, scraper, flasks
Method-
Day 1
1. Prepare all cell suspension.
2. Prepare 7 centrifuge tubes flled with 5ml of nutrient broth.
3. Inoculate the pathogens into each nutrient broth.
4. shake in incubator 30C, 200 rpm, 24 hours.
For marine bacteria suspension (grow again the bacteria of 11 and 33 from the flasks into new flasks):
1. Inoculate the bacteria into 5ml marine nutrient broth.
2. Shake in incubator 30C, 200 rpm, 24 hours.
Day 2
1. Clean the laminar flow and get it ready for the next experiment.
2. Materials needed are 14 petri dishes and labeled with the names of the seven pathogens with two petri dishes for each pathogen and also divided the petri dishes into four compartments.
3. First, spread the petri dishes with pathogens on the agar surfaces by using hockey stick.
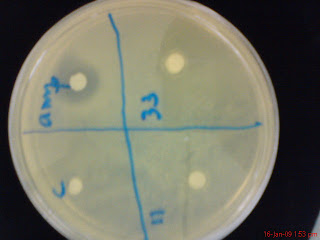
4. After that, put the disk paper into four different compartments, with each different solution for each compartment- C is for control, Amp is for ampicillin, 11 is for bacteria 11 and 33 is for bacteria 33. Do the same for the rest of the petri dishes.
5. Store the petri dishes in fridge 2-4 C for 24 hours (pre-diffusion techniques)
Day 3
Take the petri dishes out and store in ambient temperature.
Day 4
Observe result
Result:
The seven pathogens are:
1. Strep. Feacalis
2. Macro Luteus
3. proteus Ugeris
4. Entero Unerogeres
5. Strep yerus
6. E. Coli
7. Se. macro
The antibiotic sensitivity of the bacteria will be based upon the presence of halogen zone around the disk filter paper in the bacteria's compartment. In other words, if the bacteria has antibiotic sensitivity, it should have the same result as the ampicillin.
The pictures above are in the sequence of the list of pathogens above. The result, as you can seen in the pictures, most of it has been contaminated. There is only one bacteria that shows antibiotic sensitivity and that is Bacteria 33 to pathogen Se. macro. However the result is not so clear indicated as it might also be the result of contamination.
Conclusion: There is only one bacteria that shows antibiotic sensitivity and that is Bacteria 33 to pathogen Se. macro. However the result is not so clear indicated as it might also be the result of contamination.
Posted by SunSeT at 10:01 AM 0 comments
January 12
Title of Experiment: Culturing Bacteria into Nutrient Broth
Objectives: To testify the existence of samples of mucus given and to transfer the bacteria sample from petri dishes into nutrient broth medium.
Materials: nutrient broth medium in flasks, Two sample of culture microbe (labeled as 11 and 33), inoculating loop, parafilm, aluminium foils, fire flame, ethanol, tissues and permanent marker.
*Note that the culture microbes of bacteria were chosen according to its growing rate.
Method:
1. Clean the laminar flow by spraying ethanol on its surfaces and wipe it with the tissues. Switch on the blower and light the fire flame after that.
2. Move the materials into laminar flow and put it closer/around the flame. Label the flask (sterilized) 11 and 33 with researcher's name and the date.
3. Sterilize the inoculating loop by flaming it in the fire. Leave it to cool down. At the same time. remove the parafilm from the petri dish.
4. Open the petri dish gently and place the tip of the inoculating loop gently on the surface that has growing sign (it looks like white lines with gelly like on it) . Streak the tip on it slightly.
5. Close the petri dish and opent he sterilized flask immediately and put the tip of the inoculating loop into the nutrient broth immediately. Stir it if possible. Close it back with new aluminium foil.
6. Flame the loop immediately.
7. Repeat step 3-6 for the other culture microbe.
8. Put the flasks to shaker and shake around 13,000 rpm - 15,000 rppm for 24 hours in ambient temperature.
Result: The nutrient broth turn into blown blurrish color-- growing signs of bacteria
Conclusion: The growth bacteria shows that the bacteria is active. The broth is ready to be test for its antibiotic sensitivity.
Posted by SunSeT at 9:45 AM 0 comments
Wednesday, February 18, 2009
January 8
This is continuous from the previous experiment. This is the same experiment as the previous experiment except that using different samples of mucus. There were three samples of mucus used in this experiment. The purpose is to increase the chance of having a good bacteria.
The three sample mucus were named as 33, 10 and N and each of them been made into two cultures that is 33 a, 33 b, 10 a, 10 b, N a, and N b for the petri dishes. These petri dishes are then left in ambient temperature for 24 hours. The bacteria samples from the previous experiment were kept as well so that we could choose the best bacteria samples.
Result- after 24 hours the it is as below. We decided to give it a try until next week and we will grow the bacteria into broth in the flasks.
Posted by SunSeT at 11:12 PM 0 comments
Tuesday, February 17, 2009
January 5
Compared to the days before, today I am starting the real experiment. Actually this is a very basic experiment. If I understand it differently from the way you understand it, please tell me as I am still trying to improve here.
Below is a full description of the whole process of the experiment.
Title of experiment: culturing bacteria
Goal of this experiment is to testify the existence of bacteria from the samples of mucus given. Materials: 4 marine agar petri dishes, two sample of mucus (labelled as 11 and 22), parafilm, fire flame, inoculating loop, ethanol, tissues and permanent marker
Method:
1. Clean the laminar flow by spraying ethanol on the surfaces and. swipe it with the tissues.
Switch on the blower and light the fire flame after that.
2. Move the materials into the laminar flow and put it closer/around the flame.
3. The starting process of streaking the bacteria into the agar plate--
i. first, arrange the materials into a workable order, inoculating loop near the flame, 4 petri dishes on the side and mucus sample in the middle. Label the petri dish as 11a, 11b, 22a and 22b
ii. Sterilize the inoculating loop by flaming it using the fire and leave it to cool down.
iii.Open the parafilm of the mucus bottle and the petri dish.
iv.Open the cover of the fish mucus bottle gently and then flame the mouth of the bottle with fire.
v. Place the tip of inoculating loop into the bottle and make sure that the tip of the inoculating
touches the mucus.
vi. Take out the inoculating loop from the mucus bottle gently, open the petri dish, flame it a bit and streak the mucus into 1 quarter of the surface of the petri dish.
vii. Cover the petri dish immediately after streaking. Flame the inoculating loop thoroughly
and let it cool down.
viii. Repeat step iv to step viii until there are four different streaking strokes in the same
petri dish.
ix. Do the same for the rest of the petri dish/mucus samples.
4. Close the petri dish, bottle (mucus) with parafilm and put the sample back to the fridge
immediately.
5. Put the 4 petri dishes in ambient temperature or environment and leave it for 24 hours.
Result: After 24 hours, there isno clear growth sign seen. Experiment fail.
11 a - no sign of growth
11 b- no sign of growth
22 a- no sign of growth
22 b- no sign of growth
Conclusion: The reason of failure of this experiment could be that the mucus sample that we are using is not fresh enough. I learn that next time I should use fresher mucus for experiment.
Posted by SunSeT at 11:16 AM 0 comments
January 2
Kak Atirah started teaching me the method of streaking agar to the Petri dishes using inoculating loop today. There are four steps to streaking agar to the Petri dish. Before starting, I should wear the lab cloth, put on glove, get my sample (mucus), inoculating loop, Petri dish (with marine agar), fire source and parafilm. First I am to clean the laminar flow by spraying ethanol 17% into it and wipe it with tissues paper. After that, switch on the blower, lower down the glass window and put all the stuff that I needed into the laminar flow. Then, I am ready to begin the first step of streaking bacteria into the Petri dish. Kak Atirah did all the four steps for me to see first. After that, I am to do one round for her to see.
First, I am to get the inoculating loop and sterilize it by flaming it using the fire. After leaving it cooling down for awhile, I am ready to put the tip of inoculating loop into the little bottle that contains the mucus of the fish, but I should open the mouth of the bottle first. The cover and the mouth of the bottle also should be sterilized by putting it near the fire and flaming it for awhile. After that, I am to put the tip of the inoculating loop that I had sterilized just now into the bottle and dilute it with the mucus liquid. At the same time, I am to get ready the Petri dish with marine agar. Place the tip of the inoculating loop on the surface of the agar and start the first step of streaking process. The secret to streaking the bacteria to the surface of the agar was to make sure that the tip of the inoculating loop is been placed gently on it and then start by making lines. After that, flame the inoculating loop again and start the same process by putting it into the bottle of mucus and ensure that the opening of the Petri dish and the bottle are not too long till it is possible for it to catch foreign elements from the air that might carry other kind of bacteria. It will ruin the purity of the result if that ever happen. The same step continues until there are four different streaking in the same Petri dish. And then, flame, seal with parafilm. Put it into the appropriate temperature/ environment and wait for the bacteria to grow. Some of the bacteria are to be place under ambient temperature but some are to be placed under 37C temperature, dependent upon the purpose of the experiment.
Posted by SunSeT at 9:43 AM 0 comments
Monday, February 16, 2009
January 1, 2009
Today is of no holiday, not like the other places around the world. That is what I heard. Johor does not celebrate holiday on this day. So today I still come to the Bioinformatics lab as usual. I hasn’t read much of the thesis that I gotten from Kak Atirah, as there are so much things to refresh about, such as the DNA extraction, what is inoculating loop, laminar flow, test tube and even I could not remember the name for ‘bikar’ in English! Things are so blur in my head. However, I do still remember a little bit of pieces here and there about Biology. Now it is up to me to make it come back to my mind or not.
There isn’t much thing done today, except for getting ready medium and instruments ready for my project. By this I mean sterilizing everything that I need to use. First I need to prepare marine broth and as well as marine agar by using the sea water which they gotten back from the last visit (which I don’t know how long ago). This is done like this:
Materials: nutrient broth (4g for 500 m l) and nutrient agar powder (20g for 1 liter), 2-3 short bottles, scapula, plastic weighing dish, sterilize machine
1. First, weigh both the nutrient broth powder and the nutrient agar powder to its rightful weight by using the ‘weighing machine’ that only weigh in the matter of gram.
2. Next, pour the nutrient broth powder into the 1liter bottle and nutrient agar into a flask and then cover it with aluminum foil.
3 After that, putting all the other instruments that need to be sterilized, such as extra flasks (cover with foil first), forceps, and etc into the sterilizing bucket.
4. Place the bucket into the sterilize machine and make sure that the liquid medium does not mix together with the solid instruments.
5. Autoclave under 120C for 1 hour.
Left: Marine Nutrient Agar been poured into the petri dish Right: Marine broth
Posted by SunSeT at 11:49 AM 0 comments
December 31
Its the last day of 2008! I arrived C08 Bioinformatics lab about 8 in the morning. To my disappointment there is no one here! The sign on the door said that the lab will be open at 830am. Feeling so hungry, I tried to find food to eat but unfortunately could not find anything. There is no stalls around this area. I heard that there is a food restaurant nearby but to say near it still needs another long walk! AHH! I would rather stay and wait...
Dr Shahir finally appear about 9am :) Meeting him was really unexpected-I felt like I am not ready to meet him as I only communicated with him through emails and never met him face to face. From his facebook picture he looks kind of big but when meeting in person it is nothing like that at all :)
First I updated him with things in Mission College and about Dr Joy. It will really be exciting if these two professors meet one day :) But is that possible? Ask Dr Shahir go to Mission College? What will he be doing there? Or maybe ask Dr Joy to come and have a visit here:) Yeah... its a wonderful place to visit! Because it is so much bigger than Mission College! Yeah Dr Joy, you should come and visit :)
Dr Shahir showed me around the building, bring me to visit the lab, introduced me to the students (degree, masters and PhD students) and to the supervisor- Kak Atirah that will be supervising me in my project. I am not so clear what I need to do yet. All I know is that I need to be good friends with marine bacteria, hope that it won't make my job harder :)
After that, I sat down with Kak Atirah as she go through with me briefly my project and she also given me a copy of her thesis with a topic that is similar to mine. So I was told to read her thesis and to get familiar of the procedures, methods, materials, and etc of the project, because I will be doing another thesis of my own! And I never written a thesis before!
Ponders about year 2008- IF talk about reflection, all I want to say is that I am out of Mission College! How excited! yes! Well I just couldn't wait to graduate :) And I am glad to be able to explore a new place before I step into the world of work :) May this new year of 2009 be a pleasant and exciting one :)
Posted by SunSeT at 11:31 AM 0 comments
December 30
Today was a sunny day! I arrived UTM around 10 am in the morning. This place is much bigger than I thought. It looks like a big town and Mission College is only 1% of it. The place that I need to go is called C 08. Mind you that C 08 is referring to a building, not a classroom or a office. UTM is so big that they need to name each of the building according to the alphabetical order. And under each of the alphabet there is numbers beside it to represent the block (I think). So if you want to find C 08 first you need to walk through A and B.
After a few round of getting lost, finally C08 is right in front of my eyes! Now I need to find the Bioinformatics Lab and look for a girl called Ms Izzati. Meeting this girl was like out of expectation. I guess I am still shock to see the people in Malaysia... I just not used to hearing Malay language (instead of Thai language) around me and seeing Malay people (instead of Thai people) too!~ Sigh to say I am having cultural shock with my own country!
Ms Izzati led me to the place that I am going to stay. It is in Kolej Tun Doktor Ismail (College of Tun Doctor Ismail) or KTDI. You see, there are 17 colleges with similar names (either KTDI, KTHO, KTR and etc) and each of this college consists of boys' hostels and girls' hostels and these colleges hold about 25,000 students. You can imagine these hostels are. The rent for my room was Rm4 per day which equals to 40 baht includes electricity and water. Cheap right? My hostel is only about 15 minutes walk away from C08, which is good because I don't have to worry about being late to the lab :)
Dr Shahir was not around yet. I am going to see him tomorrow.
I just spend the time to unpack all of my stuff for the rest of the day. Been an international student for so many years has enabled me to do it professionally!
Posted by SunSeT at 10:56 AM 0 comments